Serendip is an independent site partnering with faculty at multiple colleges and universities around the world. Happy exploring!
Diffusion
FROM RANDOM MOTION TO ORDER:
DIFFUSION AND SOME OF ITS IMPLICATIONS
"...we know all atoms to perform all the time a completely disorderly heat motion, which, so to speak, opposes itself to their orderly behavior and does not allow the events that happen between a small number of atoms to enrol themselves according to any recognizable laws. Only in the co-operation of an enormously large number of atoms do statistical laws begin to operate... All the physical and chemical laws that are known to play an important part in the life of organisms are of this statistical kind; any other kind of lawfulness and orderliness that one might think of is being perpetually disturbed and made inoperative by the unceasing heat motion of the atoms..." -Erwin Schrödinger, What Is Life?, 1944
Most people think of stability and order as the norm, the way things are... and of movement and change as needing some explanation. Imagine a quiet lake in the forest, so still that the trees on the banks reflect downwards in it like a mirror. If a fish jumps or you throw a pebble or the wind blows, the water ripples distoring the reflected image. Undisturbed, it seems absolutely motionless, with a stability and order like glass.
In fact, as Schrödinger pointed out, it is not movement and change which requires explanation, but rather stability and order. What appears to us as a placid lake consists instead of an enormously large number of very, very small water molecules all in continual motion, every one moving relatively independently of every other one.
That this is so not only of a lake, but of every other entity (including ourselves), represents a major advance in human understanding brought about by physicists in the late nineteenth and early twentieth centuries. It is an understanding with wide-ranging, and still to be explored, implications for physics, chemistry, and biology, and with at least metaphorical implications for thinking about a host of human phenomena. What phenomena of stability and order that we think we understand might better be understood in terms of underlying continous changes of constituent parts? What new insights might follow from reorienting one's thinking in that way?
Let's take a closer look at a simple example of an apparently stable and orderly system, and the underlying continuous motion of its constituent parts. How can continuous motion lead to an appearance of stability and order? What properties do such systems have that aren't present in systems that display order and stability in the absence of movement? What properties do systems displaying "dynamic order" have that aren't present in systems displaying "static order"?
You can look into these questions below and make your own observations using the applet here (opens in a new window).
|
Starting simple: from one moving part to many
|
To appreciate stability and order based on underlying change, one first has to believe there are things changing, and then to see that certain kinds of stability and order emerge as the number of changing things increases ("statistical laws begin to operate", as Schrodinger put it).
Let's start with a single moving particle in a large box. A single particle goes about its own business, following a straight line until it hits a wall of the box, bounces off, and follows another straight line until it hits another wall. That's not so suprising or interesting. What's more significant for us is that sometimes there is a particle in one half of the box and no particle in the other, and vice versa. The "percentage" of total particles in each box changes intermittently. In the case of a single particle, each side changes from 0% to 100% as the particles crosses from one side to the other.
|
Click clear, then move the number slider to 1 and click add particles.
|
What do you suppose happens as we add more and more particles (obeying the rule that the intial trajectories of each are different)?
There continue to be fluctuations in the proportion of particles on each side, as individual particles follow their own trajectories, but the fluctuations are not as large (we never see all the particles on one side) and the state of the box over time remains close to equal proportions of particles on both sides. You should convice yourself that this gets even more so as one further increases the number of particles involved.
|
To add more particles, increase the number slider and then click on add particles. 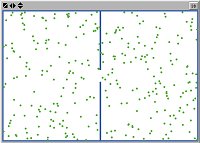  |
Its important not only to realize that adding things each of which changes can make some other things change less, but to have a feeling for why that happens. If each of the particles followed exactly the same trajectory, it wouldn't happen. Instead, we'd still be in the situation of abrupt changes from all the particles on one side of the box to all the particles on the other. What's important is that the particles follow different and unrelated trajectories. This means that, at any given time, each has the same probability of being in either the left half or the right half of the box. And so, on average, one finds roughly half on one side and roughly half on the other at any given time. What's staying more or less constant on each side of the box is not any particle, each one is still moving back and forth, but rather the collective property of the proportion of particles (whichever ones) on one side of the box and the other. That's Schrödinger's "statistical kind of lawfulness and orderliness". And, far from it "being perpetually disturbed and made inoperative by the unceasing heat motion of the atoms", it very fundamentally depends precisely on that motion.
|
To follow a single particle, click on follow/don't follow. You can get back to the regular view by clicking on the same button again.
|
So...
For some things (all?) that appear stable, there is underlying motion and change.
- The motion of water molecules in a quiet lake occurs over time and space scales outside the range which our nervous system are capable of detecting. Within our range, there is only the average ... and hence apparently unchanging behavior.
For such things, it is as much apparent stability that requires explanation as it is change, since underlying components are always changing.
It is not only quiet lakes, but also chairs, tables, people, and so forth that don't seem to change (to varying degrees). Why is that? Are they all examples of the same kind of stability or are there different kinds?
For some things, apparent stability results from statistical averaging of ongoing changes in the parts. For this to happen, its important that the parts be, in some ways, doing different things.
- Lots of things doing the same thing will not reduce and may even enhance some kinds of more global changes. For order, of the kind being considered here, it is critical that different things be changing in different ways.
|
From stability to change
|
So, we have an easy way to get at least roughly equal numbers of particles on both sides of the box. Why would we want that? Well, we might or might not, but, in any case, diffusion (the technical name for what we've started exploring) happens all the time, and its a good idea to know why. Put a gas into one side of a box, and it fills both sides equally ... open a bottle of perfume and it can shortly be smelled throughout a room ... put a spoonfull of cream in a cup of coffee and the whole cup eventually gets lighter, even if you don't stir it ... and so forth. We'll see, as we go on, that there are a whole host of physical, biological, and even social phenomena which make sense in terms of diffusion. Equally importantly, what we've got to this point is a basic driving force, the second law of thermodynamics, which says, at a very fundamental level, why things change: they go naturally and inevitably from statistically less probable to statistically more probable states. As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life.




Comments
module 1 assignment
This was a very interesting story and would have to say I never thought about certain things that cells do and the way they are in constant movement, even though it could be a solid item. Also love the idea about the whole perfume thing. Never really put much thought to it and this article has opened my eyes to things and now when I see the ponds here in Florida i will now start to think that its in constant movement. I can see where Scientific Method has been used but i see this as more informational and there for we move from less probable direction to a more probable direction in my opinion.
module 1 assignment
Wow, this one actaully educated me on the simplest of things. I liked the water in a pond story, how to the human eye it seems all is still but in truth its constant movement. I dont know about the whole Scientific Method being used in this one. I would have to say that this is more informal than anything. I would also have to say that I think that it is logical due to the fact that since he broke things down in a simplistic format i can now imagine what it is made up of which in turn moves us from less a probable direction to a more probable direction.
Module 1 Assignment
- Does the exhibit demonstrate the Scientific Method? Pay close attention to the final conclusions. "From Stability to Change." Based upon data, is the author's statement a logical one: "As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life."?
I believe the exhibit demonstrates part of the Scientific Method. After reading the article, I remember during my readings in chapter 2, discussing the arrangements of electrons and atoms. Electrons are hard to see with the naked eye but if stoped in place, each electron has a specific location and can be seen. The chapter uses the example of a ceiling fan, although it's hard to see the blades while the fan is in motion, if stopped the blades become easily visable and stop in their location. The author explains how molecules and atoms move about freely, however the movement is not easily seen with the human eye but if slowed or at a standstill the matter is easily visable.
I have gained more knowledge of molecules and atoms movement after completing the assignment.
Alicia Boyd-122433
Week 1 Assignment
The exhibit demonstrated part of the Scientific Method, some of the missing parts were in testing the hypothesis and comparing data. The model itself was very interesting, but I had to read the article multiple times to be sure I understood the purpose of the experiment. The conclusion in my opinion was not logical, the examples sited in the conclusion made more since in relation to explaining diffusion than did the model. I was a little confused on what point was being made or proven.
Module 1 Assignment
The author did make an observation which was that with what appeared to be a motionless surface was actually very small molecules in a continuous motion. He asked several question in this regard as well. He then used prior knowledge of the subject from Erwin Schrodinger to base his hypothesis off of. His prediction was that if the molecule followed the same path then there would be abrupt changes such as a ripple in the water or a wave. However if they all move separately and in different directions this gives the appearance of a motionless surface. He tested the his hypothesis with the box diagram and showed that the molecules will move in separate directions and stay spread out evenly in the box. He collected his data and interpreted his results, so in my opinion he did in fact use the Scientific method. He may not have had the best format of showing this but it is all there.
Week one
Yes this was a good example of scientific method.He began with an idea, and then took us through the steps of the experiment to form a conclusion. The conclusion was logical and brought all the information together in a way that was easy to understand.
Week 1 assignment
Yes, I do believe this article is an example of the Scientific Method. There were explanations of the hypothesis, which was a logical but unproven explanation for a given set of facts. There was a theory and a law that they described using they hypothesis. Describing the probability and movement.
Week 1 Assignment
Yes, the scientific method was demonstrated in this exhibit. The processes of the scientific method was utilized. A valid conclusion was developed after the author developed a hypothesis,and conducted the experiment. The authors statement was valid due to the fact that it the movement of many unorganized, complex functions that are present in all living matter that works together to continuously keep the organism alive.
Module 1 Assignment
This was a good example of the Scientific Method. He made observations, ask questions, took prior knowledge and formulated a hypothesis. The prediction was tested, the data was interpreted and a conclusion was discussed in a way many people could relate to. I thought this was a very thought provoking article and experiment.
NM
WEEK 1
Yes, the exhibit demonstrates the Scientific Method. It starts with the hypothesis that continuous motion and change leads to stability and order. The experiment is conducted by looking at particles in a box and their movement and change. The data gathered from the experiment is analyzed and the particles’ trajectory is discussed and compared. Finally there is an organized conclusion made. I think that the author’s conclusion seems logical.
Rebecca McGinty
Module 1 Assignment
Yes, this module's assignment does demonstrate the Scientific Method. The hypothesis was clear and logical. The un-trained person would believe that they lake is still, however it is not. There are molecules constantly moving to keep the water in its liquid state. He made an observation, came up with a question, made a hypothesis, experimented and then made a conclusion. This was a neat assignment.
WEEK 1 Assignment
Does the exhibit demonstrate the Scientific Method? Pay close attention to the final conclusions. "From Stability to Change." Based upon data, is the author's statement a logical one: "As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life."?
Yes this exhibit does demonstrate the Scientific Method. The observations are clear and the hypothesis has been met. To the human eye the particles look still but really they are moving. This was very interesting to learn about.
Week !
The exhibit was amazing to me. I think the exhibit demonstration does exhibit the "Scientific Method". The example of a still lake in the forest was a good example of being still yet moving. The environment contributes to the movement of stillness as well. I think the only thing that is certain is change and that the environment around contributes to change.
Yes I believe that the use of
Yes I believe that the use of the scientific method was used. An observation was made followed by questions that were asked about the research. After reviewing all of the information a hypothesis was made. Life is crazy and unpredictable and never knows where it is going to take you.
week 1
This does show the scientific method to a certain degree. I think that the article asks alot of questions but it also answers the questions right after. It doesnt show any sort of theory.
Module 1
I was already intrigued by the first thing the author mentioned, even though something looks still, is it really? Very interesting. I feel like the scientific method was used. The author presented us facts, asked certain questions, and then tested them out. I really like articles like this that immediately make you think and consider much more than what is really being asked.
Moduel Assignment Week 1
The above experiment did show use of the scientific Method. The author asked questions, formed n hypothesis, analyzed the data and used interactive examples to help form a conclusion. This assignment did help me better understand atoms and molecules.
Reply to Module 1 Assignment
I agree with you completely and I, too, have a better understanding of the movement within molecules and atoms. This assignment was very helpful and made it easier to comprehend. Science has never been a favorite subject of mine but with excercises like these, it has made biology more interesting.
Alicia Boyd-1122433
Week 1 Lecture
This does show the scientific method to a degree. It does pose questions but doesn't form a hypothesis to test. Instead, it tests the questions asked. So it does show a bit of the method, but not the whole thing.
Module 1 Assignment
I feel that the scientific method does also demonstrate the scientific method. I didn't realize that molecules and atoms move so freely whereas I would actually see the object at a more stand still view. However, beneath the surface those particles are yet moving and will do so even more when it comes into contact with heat.
"The Scientific Method
The exhibit demonstrates the Scientific Method. It used all the steps in the process. It developed the hypothesis, conducted the experiment, analyzed the data, compared the results, and determined a conclusion. The author used the scientific method to teach us about movement and diffusion. It explained when it was a good thing and when it was not.
Question Does the exhibit
Question
Does the exhibit demonstrate the Scientific Method? Pay close attention to the final conclusions. "From Stability to Change." Based upon data, is the author's statement a logical one: "As we'll see, it is this movement which life captures and makes use of to move itself in the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life."
Yes. In the author’s statement, he makes logical observations of movement. The Scientific Method is also defined by the author asking questions about movement and life. He formulated a hypothesis from previous findings. He also tested this with experiments and made a clear conclusion, as quoted.
Interactive Assignment Week 1
The author did use the scientific method to draw his conclusions. I had to read the essay several times to make sure I understood it. The grammar and sentence structure were so poor that I had trouble following parts of it, for example:
"Its important not only to realize that adding things each of which changes can make some other things change less, but to have a feeling for why that happens." This sentence made no sense to me. In regard to his final conclusion, I was uncertain if "less probable" was the same as or equal to "disorder".
I read the "Second Law of Thermodynamics" link and I understood it to mean the opposite, but I am unsure.
After this module, I realized I have much to learn about biology! I did enjoy the interactive experiment, which allowed me to test the laws of diffusion.
Moduel 1
I do feel that the author did follow the scientific method and that they presented the information in an informative way. By clicking on the link provided in the text, a view of what the autor was trying to say comes through, making the final point clear. With the use of the visual aid, I would have to say that the statment is a logical one.
This is a good, informative exercise.
Bio 1100 Assgn 1
Does the exhibit demonstrate the Scientific Method?
While reading this assignment and doing the experiment on my own, I feel that the author did use the Scientific Method. From the beginning he made you think and dive into his question of 'was stability the norm'. Through the experiment he was able to show us that it was in fact the movement that was the norm and that all living things are constantly in some type of motion or they would not be 'living'. I was very intrigued to see that heat actually did to the cells also. I played around with the controls and noticed that the cells speed up at the higher levels of heat. I wonder if that is why we sometimes feel faint when we get hot, because or cells are moving so rapidly. Well, the answer is yes, I believe that he effectively used the scientific method even though it was was not directly laid out the way that it was step by step in the text.
Pay close attention to the final conclusions. "From Stability to Change." Based upon data, is the author's statement a logical one: "As we'll see, it is this movement which life captures and makes use of to move itself in the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life."
I think that the author's statement was logical because it draws on the fact that 'all things must work together in order to form something that is functional'. That statement is mine but to me helps me to make logical sense of what the author is saying. It cells are not working together by bonding and being in constant movement, things like out skin, or water, or trees, really would not exist as we know them but would take another form that may not being in exact order to be useful.
Module 1 Assignment bio 1100
Yes, the author did demonstrate the scientific method by making an observation, asking question, looking at prior findings and knowledge, formulating a hypothesis and drawing a conclusion.
Absolutely, the author’s statement is a logical one because it gives us an example that we all can understand and have performed on a given day or at one point in our life, especially with the perfume and coffee example.
Module 1 assignment
In my opinion, this article is more informative. I can see how he incorporates the Scientific Method, asking questions and experimenting, etc. I like the box idea, but I personally understand better when the example is something real. Like at the end when he uses the example about cream in coffee or perfume fillig the air, because when I think of diffusion I don't think of dots in a box per say, but (like in the book) dye in water dispersing itself over time.
The statement the author makes is logical because we can understand and explain how objects are what they are. In other words, when we know microscopically what objects are made up of we can understand them better.
Moduel 1
This assignment really helped me understand how the particles move in different conditions. Experimenting myself was fun and very educational!
moduel 1 assignment
Does the exhibit demonstrate the Scientific Method? Pay close attention to the final conclusions. "From Stability to Change." Based upon data, is the author's statement a logical one: "As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life."?
The author in some aspects does demonstrate the Scientific Method, he made an observation and he asked a question. He looked at prior findings and formulated a hypothesis from the prior findings. Also in the “From Stability to Change” he make a prediction tested the hypothesis, collected and interpreted data also in the end he drew a conclusion that “As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life”.
The author's statement: "As we'll see, it is this movement which life captures and makes use of to move itself it the opposite direction, from less probable to more probable, from disorder to all the organized complexity we think of as life." A logical statement the reason is that because life does move us in directions with the events that happen to us in life, there for we move from less probable direction to a more probable direction.
This moduel asignment does
This moduel asignment does demonstrate the Scientific Method. The hypothesis could be that a person believes that if the particles are among more heat, that they will speed up. In the process of observing the experiment, one could test the hypothesis and collect his or her data after the experiment to draw the conclusion that the particles DO speed up when heat is added into the box.
moduel 1 assignment
I understand the article, he explains the movement of the box,
he also tell us how he put everything in detail order. their is atoms to perform
a completely disorderly heat motion. and yes he do demonstrate the scientific
method.
in this module assignment i
in this module assignment i learned the basics of atoms and particles and molecules. When we look at any ordinary thing we might view it as non moving but deep below all that smaller than what the human eye may see there is a world of random motion.
Module 1 Assignment
In this article, we learned about particles and atoms that make up certain things. At the begining of the article, it uses the example of a still lake. To the human eye, it looks as though there is no movement what so ever. In reality, there are particles moving in continuous motion. The rest of the article goes on to explain in better depth the movement of a particle using little dots in a white box. I do understand this subject better having read this.
moduel 1 assignment
I understand this subject matter a little more after readuing this artickle. I do think that he does follow the scentific meathod in his explanation. There is a observation, a question asked, he loooked at proior findings and ,and a prediction.
Post new comment