|
Proposal
Atomic Force Microscopy
Eden McQueen (Chemistry) and Simona Radu (Chemistry)
Atomic Force Microscopy (AFM) is a very
high resolution imaging technique capable of producing
true three-dimensional images of particles and surfaces
on the nanometer scale. In AFM, a surface is scanned
with a fine tip positioned at the end of a cantilever.
The cantilever bends in response to the forces between
the tip and the surface atoms, and the magnitude of
this deflection is monitored to provide a topographical
map of the surface. This state of the art technique
provides important information about chemical and
physical phenomena at surfaces, facilitating understanding
of abstract concepts in a tangible way. For example
the high resolution of the microscope enables students
to actually see atoms, not just imagine them. Also,
many physical and chemical relationships at the surface
of solids are intimately related to the details of
the surface topography.
During the time allotted for the project, with the
help and mentoring of Dr. Jonas Goldsmith and Dr. Krynn
Lukacs, we will begin by focusing on learning how to
operate Agilent 5500 AFM recently acquired through the
college’s Sherman Fairchild grant. Our next goal
will be to incorporate the instrument in the chemistry
curriculum, so we will create a user’s guide to
facilitate the introduction of a wide range of faculty
to this instrument. The guide will be made available
to members of the college community interested in using
the instrument for their own research and teaching projects.
In order to encourage the use of the AFM, experiments
and lab activities for Chem 103/104 General Chemistry
and Chem 251/252 Research Methodology Laboratory will
be created.
The ability of AFM to provide images of the atomic
and molecular structure of surfaces can be exploited
to illustrate physical and chemical changes that often
seem abstract to beginning students. In one existing
Chem 103 experiment, students prepare gold nanoparticles
and observe the effects of ionic strength on sizes of
the particles. Students are told that the color changes
they observe are due to formation of larger size particles
without any concrete evidence to back up the claim.
One AFM experiment we will develop will use the instrument
to visualize and measure gold particle sizes before
and after the ion strength change to provide students
hard evidence for the phenomena they observe. In another
current experiment, students examine methods that artists
use for metal surface modifications (e.g.anodization).
These methods produce macroscopic changes in color,
reflectivity, etc. of the metal that are explained in
terms of redox chemistry. We will use AFM to produce
a library of surface images taken before/after electrochemical
modification so that students can see and better understand
the nanoscale alterations that lead to macroscopic changes.
To obtain appropriately smooth samples to work with,
we will use mechanical polishing techniques to planarize
metallic substrates.
For Research Methodology (Chem251/252), we will devise
an experiment that will utilize the AFM to investigate
the structure and behavior of nucleic acids. Initially,
dry samples of DNA or RNA will be prepared from buffers
at varying ionic strength. We will then directly observe,
on the molecular level, the conformational changes that
occur when the ionic strength is changed. This can be
correlated with changes in the melting temperature.
We will also attempt to observe these changes on samples
in solution. This in situ experiment, while more difficult
to carry out, will potentially be much more illustrative
as we will be able to change the salt concentration
while imaging and follow the progress of a single molecule
over a wide range of ionic strengths.
Summary
Atomic Force
Microscopy
Simona
Radu, Eden McQueen
Mentors:
Dr. Jonas I. Goldsmith, Dr. Krynn D. Lukacs
Atomic Force Microscopy (AFM) is a very high resolution imaging technique capable of producing true three-dimensional images of particles and surfaces on the nanometer scale. In AFM, a surface is scanned with a fine tip positioned at the end of a cantilever. The cantilever bends in response to the forces between the tip and the surface atoms, and the magnitude of this deflection is monitored to provide a topographical map of the surface. This state of the art technique provides important information about chemical and physical phenomena at surfaces, facilitating understanding of abstract concepts in a tangible way. For example the high resolution of the microscope enables students to actually see atoms, not just imagine them. Also, many physical and chemical relationships at the surface of solids are intimately related to the details of the surface topography.
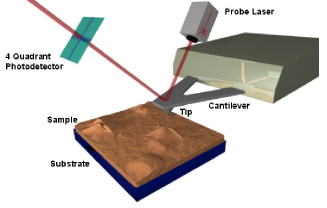
mrsec.wisc.edu/Edetc/nanoquest/afm/images/afm.jpg Ó
During the course of our internship we familiarized ourselves with the set-up and experimental procedure associated with atomic force microscopy. The microscope functions in two modes, “contact” mode and “tapping” mode. We used these on a variety of samples: organic, inorganic, and biological. Samples scanned include a synthetic polymer, gold nano-particles, red blood cells and DNA.
After acquiring the basic skills to operate the instrument we set out to master the finer points of the imaging techniques. This proved more difficult than initially anticipated—achieving a quality image is a tedious and potentially frustrating process—but this makes obtaining a good image even more satisfying. We discovered that imaging is affected by many factors, which must be carefully controlled to obtain good results.
The main factors that must be taken into consideration when trying to get good quality images are gain, speed, vibrations and the length of time the tip has been previously used. The gain “measures” the amount of topographical details that will be shown in the picture. If the gain is too high the picture will appear extremely noisy and the tip can sometimes vibrate hard enough to break. The speed of the scanner, when it is not in an optimal range, could cause the scanner to fail in observing important changes in the topography of the sample and sometimes it can make the tip crash into the surface or drag particles around. Image processing is also essential. Below is an example of a poor image, taken at the beginning of the internship (Pic a., taken with an older tip at high speed), in contrast with a better quality image taken later, after improving our technique (Pic b., new tip, balance between gain and speed and reduction of room vibrations). Other successful images are also shown below (Pictures d through f)
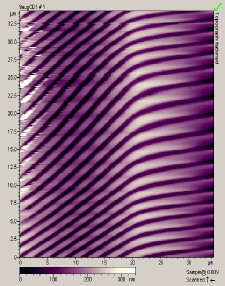
Pic. a. Bad image of a CD
|
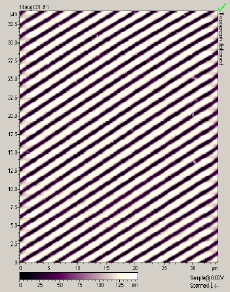
Pic. b. Good quality image |
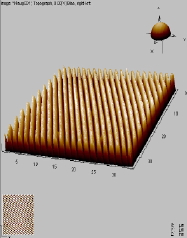
Pic. c. 3D image of CD surface |
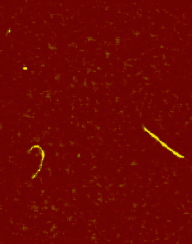
Pic. d. DNA |
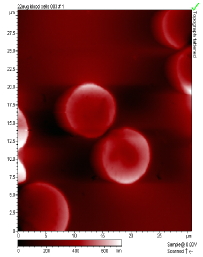
Pic. e. Blood Cells |
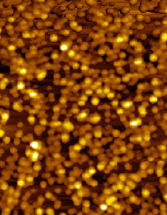
Pic. f. Gold nano-particles |
One of our goals was to generate a curriculum that could be used by l00 or 200 level science classes. While working with the microscope we realized that for lower-level chemistry students spending time to image possible samples can be somewhat tedious and time-consuming. However, the images acquired can be very useful for students to understand some abstract concepts (e.g. see if and how gold nano-particles aggregate as they are produced from reduction of gold(III) ion). There many more ways to implement the use of the microscope in higher-level courses (e.g. in Chem 251 Research Methodology the AFM could be used for DNA imaging).
We finished the internship with the accomplishment of our major goal, which was to write a user guide and troubleshooting manual for potential users (faculty, upper-level students, etc.). This worked out very well because as we familiarized ourselves with the instrument we also familiarized ourselves with common problems and sources of error, which helped us succeed in generating a useful tool for new users.
|

