So, what do I do with my computer?
Al Albano
In the past fifteen years or so, my research has come to rely almost completely on computers. My interests are in the development and testing of computational tools for the study of signals that display complexity in their spatial distribution as well as in their temporal evolution, and the use of these tools for the analysis of biological or biomedical signals.
Current projects include studies of signals propagating in the ventral nerve cord of the leech in connection with the initiation of swimming, electroencephalographic (EEG) signals from patients undergoing electroconvulsive theraphy (ECT), and EEG signals derived from subjects suffering from epilepsy. The leech work is being done in collaboration with Peter Brodfuehrer, the EEG work with colleagues at MCP-Hahneman University, Duke University Health Center, the University of Connecticut Health Center and BrainVue Inc. a neurological consulting firm based in Philadelphia.
In the following, I discuss some issues connected with the analysis of data from epileptics. This work does not address fundamental scientific issues. Rather, it asks questions whose answers might have potential as diagnostic tools. For instance, in between seizures, are there quantifiable differences between the EEG’s generated by epileptics and those from non-epileptics? In the EEG’s of epileptics, are there features that may be used as early warning signals of impending seizures? The clinical implications of affirmative answers to these questions (if they exist) are quite clear.
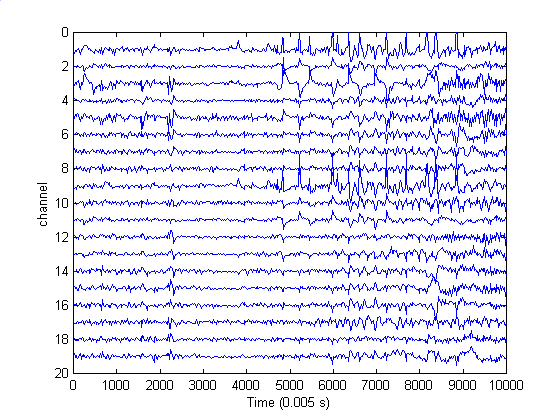
One of the approaches taken in this work is prompted by the observation that neurological function is characterized by correlated activity and a high volume of information transfer among different parts of the brain. An imperfect, but nonetheless useful, assessment of both can be obtained by examining multichannel EEG records that are measured with an array of scalp electrodes. Figure 1 shows a 19-channel record that includes an episode of epilepsy. The seizure starts at 12.0 s (point 2400), with a focus near channels 3 and 5, corresponding to the left temporal lobe. The sharp spikes from about 25 s (point 5000) to 42 s (point 8200) are eyeblink artifacts, most pronounced in channels 1 and 9 which are nearest the eyes.
 | Figure 1. A 19-channel EEG record. Seizure started at 12 s (point 2400) with a focus near channels 3 and 5. The large spikes starting at about 25 s (point 5000) are eyeblink artifacts. These are most pronounced in channels 1 and 9 which are nearest the eyes. |
A traditional statistical technique for assessing correlated activity between pairs of channels is to calculate the covariance of their signals. This quantity measures the similarity of the time courses of the signals. A slight variation of the technique estimates the similarity of the signal from one channel with that from the other after some time delay. This latter quantity could be regarded, somewhat optimistically, as a measure of the information transfer between the channels. That is, if the signal from one channel is similar to that from another channel some time later, there could have been some transfer of information from one to the other.
Figure 2 shows covariances of all channel pairs for a succession of non-overlapping 1000-point (5.0-s) intervals. Note the structures associated with channels 3 and 5, which are nearest the eventual epileptic focus These structures appear as early as in the top left panel, some 10 seconds before the clinically detected start of the seizure which occurred in the time interval covered by the top right panel. These structures "propagate" until the seizure starts in the top right panel. Note, however that the color scale is not the same for all nine panels. On the average, covariances increase greatly from the middle of the second row of panels until the end.
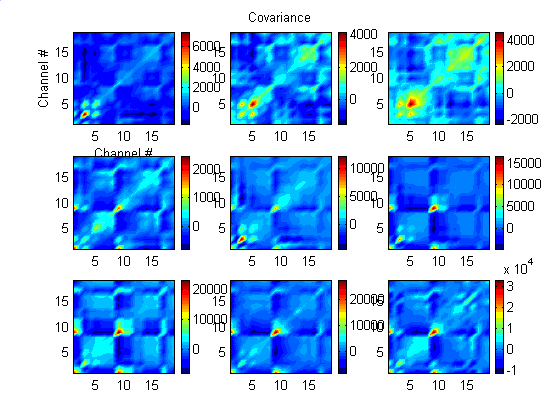
| Figure 2. Covariance among all channel pairs in nine 1000-point (5.0-s) epochs. Time sequence goes from left to right, top to bottom. The seizure starts in the top right panel. In the first panel, there is some structure involving Channels 3 and 5, which are nearest the eventual seizure focus. This propagates among other channel pairs until the third panel which includes the seizure. The color scale is not the same for all nine panels. |
A measure such as the covariance, which relies on the details of the time evolution of the signals is, however, of limited utility when dealing with signals generated by nonlinear systems. One class of such signals which has gained some recent notoriety are chaotic signals which are characterized, in part, by their so-called "sensitive dependence on initial conditions." That is, systems governed by exactly the same rules and subject to the same constraints but which start from slightly different initial conditions can have time evolutions with vastly different details. Covariance may not be sufficiently sensitive to the similarities of different trajectories generated by the same nonlinear system subject to identical constraints. On the other hand, it is very sensitive to signal variance so that two signals with large variances but which are not really well-correlated will have a larger covariance than better-correlated signals with small amplitudes. Indeed, similar calculations using the autocorrelation function, which removes the effects of the individual variances, is not very informative. It is therefore of some interest to investigate some nonlinear measures that are more sensitive to similarities in nonlinear structures and less sensitive to signal variance.
A nonlinear quantitative measure satisfying the above requirements is provided by the mutual information of each electrode pair. The average mutual information of two time series is the amount of information in one that can be predicted by measuring the other. This is a notion introduced by Claude Shannon half a century ago in the context of "Information Theory," a field he invented to help analyze questions of information transfer in telephone networks. Figure 3 shows the mutual information of all pairs of channels for the same time intervals used in Figure 2. In this case, however, there is a time delay of 3.0 ms between the channels being compared. The same color scale is used in all panels. There is a reduction in information transfer, as estimated by this metric, prior to clinically discernible seizure onset. In the case of the focal seizure examined here, this reduction first occurred in the area of the brain that was subsequently shown to contain the epileptogenic focus.
 | Figure 3. Average mutual information for all channel pairs in nine 1000-point (5.0-s) epochs. There is a 3.0 - ms delay between the channels that are being compared. Time sequence goes from left to right, top to bottom. The seizure starts in the top right panel. In the first two panels, which precede seizure onset, Channels 3 and 5, which are nearest the eventual seizure focus, have significantly lower values of mutual information. In the third panel, when the seizure occurs, the overall value of mutual information is reduced. The same color scale is used in all panels. |
Both calculations contain two clinically valuable elements: a prediction of seizure onset and a preliminary localization of the epileptogenic focus. Contrary to common lore that epileptic seizures are characterized by synchronous or near synchronous behavior of different brain sites, these results show that although the seizure is characterized by large covariances, it is also characterized by decreased levels of mutual information. The increased covariances during seizure may be more due to increases in the variances of the individual signals rather than to similarities in their structure. Whether these results generalize remains to be seen.
Return to Computers and Research